La miocarditis es la inflamación del músculo cardíaco (miocardio). La inflamación puede reducir la capacidad de bombeo del corazón y causar ritmos cardíacos rápidos o irregulares (arritmias).
La infección por un virus suele causar la miocarditis. A veces, la miocarditis puede ser el resultado de una reacción a un medicamento o formar parte de una afección inflamatoria más generalizada. Los signos y síntomas de la miocarditis incluyen dolor de pecho, fatiga, falta de aire y latidos cardíacos rápidos o irregulares.
Muchos virus se asocian comúnmente con la miocarditis, entre ellos los
que causan el resfriado común (adenovirus); la COVID-19; las hepatitis B
y C; el parvovirus, que provoca un sarpullido leve, generalmente en los
niños (quinta enfermedad); y el virus del herpes simple.
Global Reports of Myocarditis Following COVID-19 Vaccination: A Systematic Review and Meta-Analysis
Abstract
En diciembre de 2020, la FDA concedió la aprobación de emergencia a las vacunas COVID-19 de Pfizer-BioNTech (BNT162b2) y Moderna (mRNA-1273). Recientemente, los medios de comunicación han informado de casos de miocarditis después de recibir las vacunas COVID-19, en particular las vacunas de ARN mensajero (ARNm), lo que ha provocado la preocupación del público. Esta revisión resume la información de las series de casos y los informes de casos publicados, con un fuerte énfasis en la información de las características de los pacientes y la enfermedad, la investigación y el resultado clínico, para proporcionar una imagen completa de la condición. En esta revisión sistemática participaron 40 estudios, que incluían 147 casos. La edad media era de 28,9 años; el 93,9% eran hombres y el 6,1% mujeres. El 72,1% de los pacientes recibió la vacuna de Pfizer-BioNTech (BNT162b2), el 24,5% de los pacientes recibió la vacuna Moderna COVID-19 (mRNA-1273), y el resto del 3,3% recibió otros tipos de vacunas. Además, la mayoría de los casos de miocarditis (87,1%) se produjeron después de la segunda dosis de la vacuna, tras un intervalo de tiempo medio de 3,3 días. Los síntomas más frecuentes fueron dolor torácico, mialgias y fiebre. Los niveles de troponina fueron sistemáticamente elevados en el 98,6%. El ECG de ingreso era anormal en el 88,5% de los casos, y la FEVI izquierda era inferior al 50% en el 26,5% de los casos. La gran mayoría de los pacientes (93,2%) resolvieron los síntomas y se recuperaron, y sólo 3 pacientes fallecieron. Estos hallazgos pueden ayudar a la política de salud pública a considerar la miocarditis en el contexto de los beneficios de la vacunación contra la COVID-19, así como a evaluar el estado cardíaco antes de la elección de la vacuna, que se ofrece a los adultos varones. Además, se debe sopesar cuidadosamente el beneficio muy importante de la vacunación.
Introduction
Específicamente, este informe examina la literatura actual sobre la miocarditis después de la vacunación con COVID-19, resumiendo la información disponible de informes de casos y series de casos previamente publicados, con una fuerte atención en la presentación de informes sobre las características de los pacientes y la enfermedad, así como la investigación y el resultado clínico, con el fin de proporcionar una imagen completa de la condición.
Methods
Review objectives
The main objective is to clarify the potential occurrence of myocarditis associated with COVID-19 vaccination and elaborate on the demographic and clinical characteristics of COVID-19 vaccinated individuals who develop myocarditis and how many cases have been reported in the literature.
Protocol and Registration
The review is written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines for the systematic review of available literature [3]. The protocol of the review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with ID CRD42022308997. The AMSTAR-2 checklist was also used to evaluate this study, and it was found to be of high quality [4]. This review article didn’t need to get ethics approval.
Search strategy
A comprehensive search of major electronic databases (PubMed and Google Scholar) was conducted on February 10, 2022, to locate all publications. The AND operator was used to connect two of the most important concepts in the search terminology (“COVID-19” AND “Myocarditis”). (“Myocarditis” and “COVID-19” OR “SARS-CoV-2” OR “Coronavirus Disease 2019” OR “severe acute respiratory syndrome coronavirus 2” OR “coronavirus infection” OR “2019-nCoV” AND “vaccine, vaccination, OR vaccine” were used in the search. To make sure the search was completed, we checked the references of all relevant papers.
Eligibility criteria
All case series and case reports on post-COVID-19 vaccine myocarditis in humans were included. Individuals who develop myocarditis after receiving the COVID-19 vaccine, regardless the type of vaccine and dose. The references of the relevant articles will also be reviewed for additional articles that meet the inclusion criteria. Narrative and systematic reviews, original and unavailable data papers were excluded in this review. Moreover, articles other than English were excluded in this review.
Data extraction and selection process
PRISMA 2020 was used to guide every step of the data extraction process from the original source. The Rayyan website was used by two independent authors (SKA and RAE) to screen abstracts and full-text articles based on inclusion and exclusion criteria [5]. The third author (RAE) resolved any discrepancies between the two independent authors. Microsoft Excel spreadsheets were used to collect the necessary information from the extracted data. Author names, year of publication, age, gender, type of COVID-19 vaccine, dose, days to symptoms onset, symptoms, troponin level, LVEF 50% or LVEF > 50%, ECG, length of hospital stay/days, treatment, and outcomes were extracted from each study.
Critical appraisal
To assess the quality of all included studies, we used the Joanna Briggs Institute’s critical appraisal tool for case series and case reports [6]. Each article was evaluated by two different authors (SKA and RAE), each of whom worked independently. Paper evaluation disputes were resolved either through discussion or by a third author (RAE) depending on the circumstances. Articles with an average score of 50 percent or higher were included in the data extraction process. The AMSTAR 2 criteria [4]. were used to evaluate the results of our systematic review. The AMSTAR 2 tool assigned a “medium” rating to the overall quality of our systematic review.
Data synthesis and analysis
All the articles included in the current systematic review were analysed, and the data was extracted and pooled. This included (authors’ names, year of publication; gender; type of COVID-19 vaccine, dose, days to symptoms onset, troponin level, LVEF below or above 50%, ECG, length of hospital stay/days; treatment and outcomes). We gathered this data from the results of eligibility studies. COVID-19 vaccine recipients who developed myocarditis were included in the study.
Results
Selection of studies
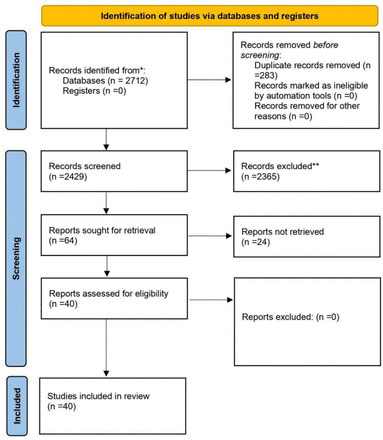
When we searched the major databases (PubMed and Google Scholar) on February 10, 2022, we discovered 2712 articles that were relevant to our search criteria. A citation manager tool (Mendeley) was then used to organize the references, and 283 articles were automatically removed because they contained duplicate content. Next, the titles, abstracts, and full texts of 2429 articles were checked for accuracy, and 2365 articles were rejected because they did not meet the criteria for inclusion. Besides that, 64 articles were submitted for retrieval, but twenty-four were rejected because they did not meet our inclusion requirements. The current systematic review was limited to 40 articles in total (Fig 1). The details of case reports and case series are shown in (Table 1).
Characteristics and outcomes of patients with myocarditis related to COVID-19 vaccine.
PRISMA flow-diagram
Characteristics of the included studies
Overall, forty studies, including 147 cases each, from the United States, Italy, Israel, Germany, Poland, France, Korea, Brazil, Japan, Mexico, and Iran participated in this systematic review. The median age was 28.9 years; 93.9% were male and 6.1% were female. 72.1% of patients received the Pfizer-BioNTech (BNT162b2) vaccine, 24.5% of patients received the Moderna COVID-19 Vaccine (mRNA-1273), and the rest of the 3.3% received other types of vaccines (Johnson & Johnson, AstraZeneca, Sinovac).
The vast majority of cases are from the United States. All patients were diagnosed with myocarditis or myopericarditis following COVID-19 vaccination, regardless of the type of vaccine and dose.
Furthermore, most myocarditis cases (87.1%, n = 128) occurred after the second vaccine dose, after a median time interval of 3.3 days. The most frequently reported symptoms were chest pain (100% n = 147), fever (46.9% n = 69), myalgia/body aches (54.4% n = 80), and also variable reports of viral prodromes such as chills, headaches, and malaise. Troponin levels were consistently elevated in 98.6% (n = 145) of the cases where they were reported, consistent with myocardial injury. The admission electrocardiogram (ECG) was abnormal in 88.5% (n = 130) of cases, and the left ventricular ejection fraction (LVEF) was lower than 50% in 26.5% (n = 39) of cases. The median length of hospital stay was 5.2 days in 127 patients but unknown in 20 patients. The vast majority of patients (93.2%) (n = 137) resolved symptoms and recovered, and only 3 patients died (Table 2).
Discussion
The current systematic review summarized evidence from the original case reports and case series that explored the development of myocarditis after COVID-19 vaccination. Throughout the selected studies, most of the participants were male, from the USA, and their mean age 28.94 was years old. The mechanism of vaccine-induced myocarditis is not known but may be related to the active pathogenic component of the vaccine and specific human proteins, which could lead to immune cross-reactivity resulting in autoimmune disease, which is one cause of myocarditis [7–10]. The occurrence of myocarditis in men may be related to sex hormone variations, as testosterone hormone suppresses anti-inflammatory immune cells while promoting more aggressive T helper cells [7,11].
These findings were matched with Oster et al (2022) [12], who found the incidence rate of myocarditis among vaccinated male people was similar to that seen in typical cases of myocarditis and there was a strong male predominance for both conditions [13]. Fatima et al (2022) [7] found most patients who developed myocarditis were males. Moreover, Patone et al (2022) [14] mentioned that the incidence of myocarditis was among England males younger than 40 years old. Similarly, a systematic review study found that the Incidence of myocarditis following mRNA vaccines is low but probably highest in males aged 12-29 years old [15].
Another important finding in the current systematic review is that most of the participants received Pfizer-BioNTech (BNT 162b2) followed by the Moderna CVID-19 vaccine (mRNA-1273), and most of the cases who complained of myocarditis received two doses of the vaccine. This indicates that mRNA vaccines are associated with a higher risk of developing myocarditis than viral vector vaccines, including Janssen, Oxford, and Sinovac. Bozkurt et al (2021) [2], have assumed that autoantibody generation could attack cardiac myocytes in response to mRNA vaccine, which increases the risk.
This finding is matched with Patone (2022) [14], who mentioned the risk of myocarditis increased within a week of receiving the first dose of both adenovirus and mRNA vaccines and after the second dose of mRNA vaccine. Oster et al (2022) [12] concluded that the risk of myocarditis after the mRNA vaccine was increased after the second dose in adolescents and young males. On the other hand, Simone et al (2021) [16] concluded that there is no relationship between COVID-19 mRNA vaccination and post vaccination myocarditis.
The findings extend these observations, which include the median onset of symptoms after vaccine administration was 3.32 days. The most common symptoms are chest pain, followed by myalgia/ body aches and fever. These findings matched with Pillay et al (2021) [15], who reported in a systematic review observation that the majority of myocarditis cases had a short symptoms onset of 2 to 4 days after a second dose, and the majority presented with chest pain. These findings matched with Oster et al (2022) [12], who mentioned myocarditis was diagnosed within days of vaccination.
In patients with severe myocarditis, the diagnosis is often established by heart biopsy. In patients with mild myocarditis, the diagnosis is based on compatible clinical findings and confirmed by elevated levels of blood markers or an electrocardiogram (ECG) indicative of cardiac injury, with the presence of new abnormalities on echocardiography or cardiac MRI [17].
Cardiac-specific investigations revealed that troponin levels were elevated in almost all of the cases, consistent with myocardial injury, which is associated with autoimmune processes matched with vaccine protein and the case immune system.
In the same line with Lee et al. (2022) [1], a systematic review to investigate myocarditis following COVID-19 Vaccination in October 2020–October 2021, mentions that all reported cases have an elevated troponin level in keeping with myocardial injury.
In our study, less than one third of cases had left ventricle ejection friction (LVEF) was less than 50%. Compared to patients with COVID-19 illness, patients with vaccine associated myocarditis had a higher LVEF.
This finding is consistent with the findings of Fronza et al. (2022) [18], who investigated myocardial injury patterns at MRI in COVID-19 Vaccine and discovered that more than half of the cases had more than 50% LVEF. Also, Shiyovich et al. (2022) [19], who analyzed myocarditis following the third (Booster) dose of COVID-19 vaccination found that the mean left ventricular ejection fraction was 61 ± 7% (range 53–71%) and regional wall motion abnormalities were present in one of the patients only. Global T1 values were increased in one (25%) of the patients, while focal values were increased in 3 (75%) of the patients. Global T2 values were increased in one (25%) of the patients, while focal values were increased in all of the patients (100%). Global ECV was increased in 3 (75%) of the patients, while focal ECV was increased in all the patients (100%). LGE was present in all the patients.
In our systematic review and meta-analysis study, 88.5% of cases had abnormal changes in the electrocardiogram (ECG) result, regardless of the vaccine type.
Vidula et al. (2021) [20] support our findings by reporting two patients with clinically suspected myocarditis who presented with acute substernal chest pain and/or dyspnea after receiving the second dose of the vaccine and were found to have diffuse ST elevations on electrocardiogram (ECG), elevated cardiac biomarkers and inflammatory markers, and mildly reduced left ventricular (LV) function on echocardiography.
Also, Puchalski et al. [21] reported the findings of a case series regarding COVID-19-Vaccination-Induced Myocarditis in Teenagers. Electrocardiogram (ECG) patterns varied, but in all cases, characteristic features of acute myocardial injury, including ST segment elevation or depression, and repolarization time abnormalities, were present.
Management of myocarditis remains largely supportive and is based on the restoration of hemodynamic stability and the administration of guideline-directed heart failure and arrhythmia treatment. Patients with preserved ventricular function and non-severe features were often treated with colchicine or non-steroidal anti-inflammatory drugs. According to our findings, all cases were treated with NSAIDs, beta blockers, calcium channel blockers, and/or diuretics. The median length of hospital stay was 5.2 days in 127 patients, and the vast majority of patients resolved symptoms and recovered, and only 3 patients died.
This finding broadly supports the work of other studies in this area. Woo et al [22] reported that many patients who received anti-inflammatory agents such as NSAIDs, colchicine, steroids, and intravenous immunoglobulin recovered without further medical treatment, with a hospital stay lasting 3-6 days.
In accordance with the present results, previous studies have demonstrated that almost all of the cases experienced a prompt recovery with no residual cardiac dysfunction. The median length of stay for all myocarditis cases was around 2–3 days, with a range of 2–10 days [23].
Conclusion
En conclusión, estos resultados pueden ayudar a la política de salud pública a considerar la miocarditis en el contexto de los beneficios de la vacunación contra el COVID-19, así como a evaluar la condición cardíaca antes de la elección de la vacuna, que se ofrece a los adultos varones. Además, se debe sopesar cuidadosamente el beneficio muy sustancial de la vacunación. Por otra parte, es necesario seguir investigando para evaluar las consecuencias a largo plazo y otros factores de riesgo tras la inmunización, en concreto de la vacuna mRNA-1273.
Conflicts of interest
There is no conflict to be declared.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Agreement Statement
We declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We confirm that the order of authors listed in the manuscript has been approved by all of us. We understand that the Corresponding Author is the sole contact for the Editorial process. He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
Data availability Statement
All relevant data are within the manuscript and its supporting information files.
Authors’ contributions
Conception and design SKA acquisition of data SKA, RAE, MGM, EAA analysis and interpretation of data SKA, MGM, RAE, EEA, drafting of the manuscript SKA, RAE, MGM, EAA, PKI critical revision of the manuscript for important intellectual content statistical analysis SKA, MGM, RAE, EEA, PKI, AAK, ZHW administrative SKA, MGM, EAA technical SKA, AAK, ZHW, supervision SKA, RAE and all authors approving final draft.
Provenance and peer review
Not commissioned, externally peer-reviewed
Acknowledgments
Not applicable
References
- [1].↵
- [2].↵
- [3].↵
- [4].↵
- [5].↵
- [6].↵
- [7].↵
- [8].
- [9].
- [10].↵
- [11].↵
- [12].↵
- [13].↵
- [14].↵
- [15].↵
- [16].↵
- [17].↵
- [18].↵
- [19].↵
- [20].↵
- [21].↵
- [22].↵
- [23].↵
- [24].
- [25].
- [26].
- [27].
- [28].
- [29].
- [30].
- [31].
- [32].
- [33].
- [34].
- [35].
- [36].
- [37].
- [38].
- [39].
- [40].
- [41].
- [42].
- [43].
- [44].
- [45].
- [46].
- [47].
- [48].
- [49].
- [50].
- [51].
- [52].
- [53].
- [54].
- [55].
- [56].
- [57].
- [58].
- [59].
- [60].
- [61].
- [62].https://www.medrxiv.org/content/10.1101/2022.03.27.22273007v1.full
Generalmente la miocarditis desaparece sin dejar complicaciones permanentes. Sin embargo, la miocarditis grave puede dañar de forma permanente el músculo cardíaco, lo que podría causar lo siguiente:
- Insuficiencia cardíaca. Si no se trata, la miocarditis puede dañar el músculo cardíaco, de manera que este ya no puede bombear la sangre eficazmente. En casos graves, la insuficiencia cardíaca relacionada con miocarditis puede requerir un dispositivo de asistencia ventricular o un trasplante de corazón.
- Ataque cardíaco o accidente cerebrovascular. Si se daña el músculo cardíaco y no puede bombear sangre, la sangre que se acumula en el corazón puede formar coágulos. Si un coágulo obstruye una de las arterias del corazón, puedes tener un ataque cardíaco. Si un coágulo sanguíneo en el corazón se traslada hasta una arteria que va al cerebro, puedes tener un accidente cerebrovascular.
- Ritmos cardíacos rápidos o irregulares (arritmias). El daño al músculo cardíaco puede causar arritmia.
- Muerte cardíaca súbita. Ciertas arritmias graves pueden provocar que el corazón deje de latir (paro cardíaco repentino). Esto es mortal si no se trata inmediatamente.
Prevención
No hay una forma específica de prevenir la miocarditis. Sin embargo, tomar las siguientes medidas para prevenir infecciones podría ayudar:
- Evita estar con personas que tengan una enfermedad vírica o síntomas similares a la gripe hasta que se hayan recuperado. Si estás enfermo y tienes síntomas de una infección vírica, trata de evitar exponer a otras personas.
- Practica buenos hábitos de higiene. El lavado frecuente de manos puede ayudar a evitar propagar la enfermedad.
- Evita comportamientos riesgosos. Para reducir las probabilidades de contraer una infección del miocardio relacionada con el VIH, practica sexo seguro y no uses drogas ilícitas.
- Minimiza la exposición a las garrapatas. Si pasas tiempo en zonas infestadas de garrapatas, usa camisas de mangas largas y pantalones largos para tapar la mayor parte del cuerpo que sea posible. Aplícate repelentes para garrapatas o para insectos que contengan dietiltoluamida (DEET, por sus siglas en inglés).
- Vacúnate. Mantente al día con las vacunas recomendadas, incluidas aquellas que te protejan contra la COVID-19, la rubéola y la gripe (enfermedades que pueden causar miocarditis). En pocas ocasiones, la vacuna contra la COVID-19 puede causar inflamación del músculo cardíaco (miocarditis) e inflamación del revestimiento externo del corazón (pericarditis), especialmente en hombres de 12 a 17 años. Habla con el proveedor de atención médica sobre los beneficios y los riesgos de las vacunas.
- https://www.mayoclinic.org/es-es/diseases-conditions/myocarditis/symptoms-causes/syc-20352539
No hay comentarios:
Publicar un comentario